Introduction
Hey there, readers! Welcome to Rintiksedu.id. In this article, we’ll dive deep into the fascinating topic of reducing activation energy in a reaction. As someone who has had their fair share of experiences with “energi aktivasi suatu reaksi dapat diperkecil dengan cara,” I’m excited to share some valuable insights with you. Before we get started, let’s set the stage with an eye-catching featured image:
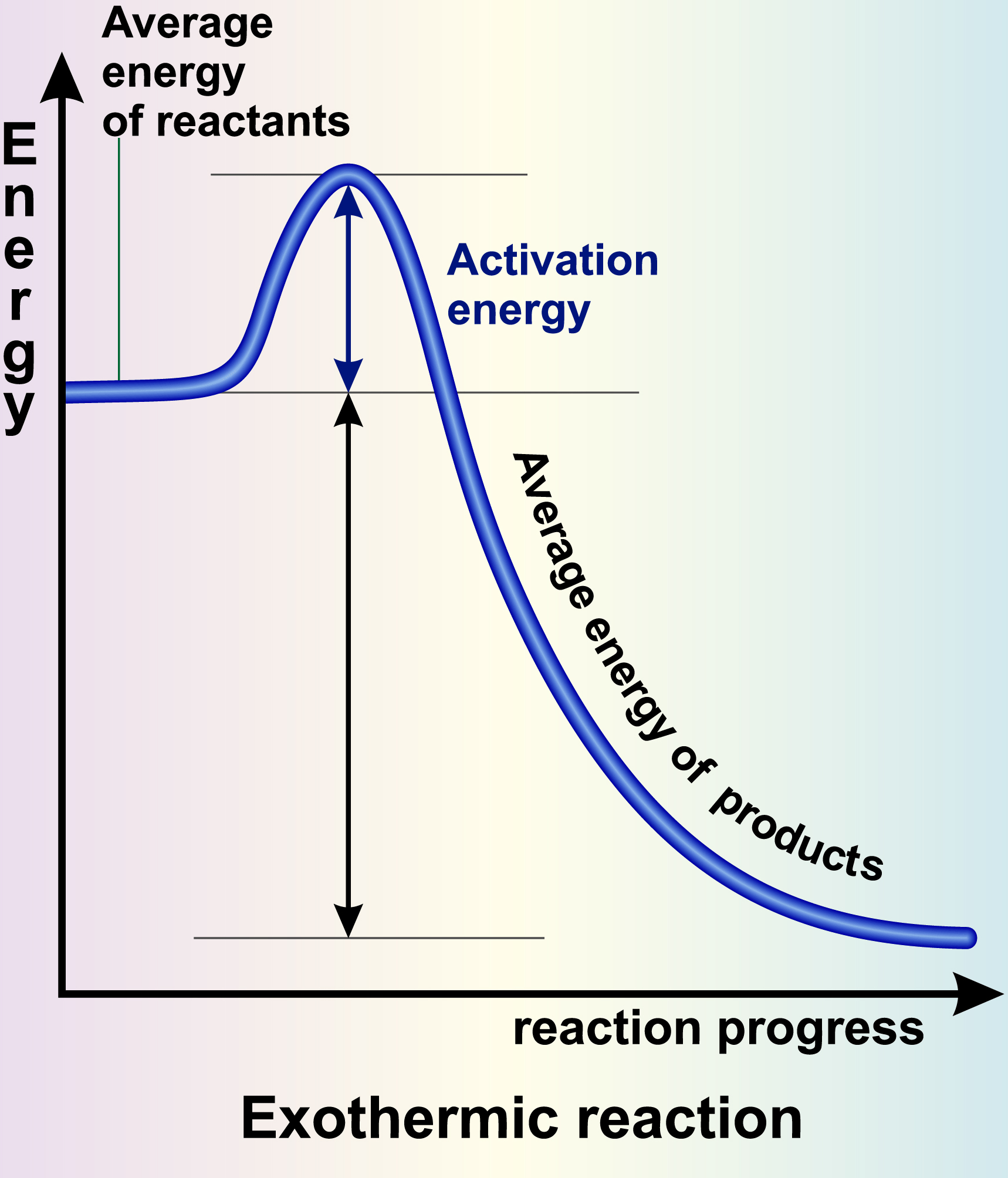
The Role of Temperature
Increased Temperature and Reaction Rates
One effective way to reduce activation energy is by increasing the temperature of the reaction. When we raise the temperature, the kinetic energy of the reactant molecules also increases. This results in faster and more frequent collisions between particles, leading to a higher reaction rate. So, don’t underestimate the power of heat in catalyzing reactions!
Catalysts: Nature’s Reaction Boosters
Catalysts are substances that can significantly lower the activation energy of a reaction without being consumed in the process. These incredible compounds provide an alternative pathway for the reaction to occur, which requires less energy. By introducing a catalyst into the mix, we can speed up reactions and achieve impressive results with smaller energy inputs.
The Power of Concentration and Surface Area
Increasing Concentration for Faster Reactions
Another effective way to reduce activation energy is by increasing the concentration of the reactants. When the concentration is higher, there are more particles available for collisions, resulting in a greater chance of successful reactions. So, remember to amp up the numbers if you want your reactions to proceed with lightning speed!
Expanding Surface Area for Enhanced Reactivity
Increasing the surface area of the reactants is yet another strategy to lower activation energy. By breaking down larger particles into smaller ones or increasing the exposed area of the reactants, we provide more opportunities for collisions to occur. Think of it as maximizing the chances of successful encounters between particles, ultimately leading to faster reactions.
FAQ
1. Apa itu energi aktivasi dan mengapa kita perlu menguranginya?
Energi aktivasi adalah energi yang diperlukan agar suatu reaksi bisa terjadi. Mengurangi energi aktivasi penting karena semakin rendah energi yang dibutuhkan, semakin mudah reaksi terjadi dan semakin cepat laju reaksi tersebut.
2. Bagaimana meningkatkan suhu dapat mengurangi energi aktivasi?
Dengan meningkatkan suhu, energi kinetik molekul reaktan akan meningkat. Hal ini menyebabkan tumbukan antar partikel menjadi lebih sering dan lebih cepat, sehingga laju reaksi meningkat.
3. Apa itu katalis dan bagaimana cara kerjanya?
Katalis adalah zat yang dapat menurunkan energi aktivasi reaksi tanpa dikonsumsi dalam proses tersebut. Katalis memungkinkan reaksi terjadi melalui jalur alternatif dengan energi yang lebih rendah, sehingga mendukung terjadinya reaksi dengan lebih efisien.
4. Bagaimana meningkatkan konsentrasi dapat mempercepat reaksi?
Dengan meningkatkan konsentrasi reaktan, jumlah partikel yang saling bertumbukan akan lebih banyak. Ini akan meningkatkan peluang terjadinya reaksi yang sukses, sehingga reaksi dapat berlangsung dengan lebih cepat.
5. Mengapa memperluas luas permukaan dapat meningkatkan reaktivitas?
Dengan memperluas luas permukaan reaktan, kita memberikan lebih banyak area yang terpapar untuk bertumbukan. Hal ini memberikan peluang yang lebih banyak bagi partikel untuk berinteraksi dan bersentuhan, sehingga reaksi berjalan lebih cepat.
Conclusion
In conclusion, reducing activation energy in a reaction is key to increasing the reaction rate and achieving desired outcomes more efficiently. By employing techniques such as increasing temperature, using catalysts, and optimizing concentration and surface area, we can lower the barrier for reactions to occur. So, remember to apply these strategies wisely in your chemical endeavors! Thank you for joining us at Rintiksedu.id, and we hope you found this article enlightening and empowering.







